- Renishaw Plc Port Devices Driver Download
- Renishaw Plc Port Devices Driver Updater
- Renishaw Plc Port Devices Drivers
- Renishaw Plc Port Devices Driver Windows 7
- Renishaw Plc Port Devices Driver
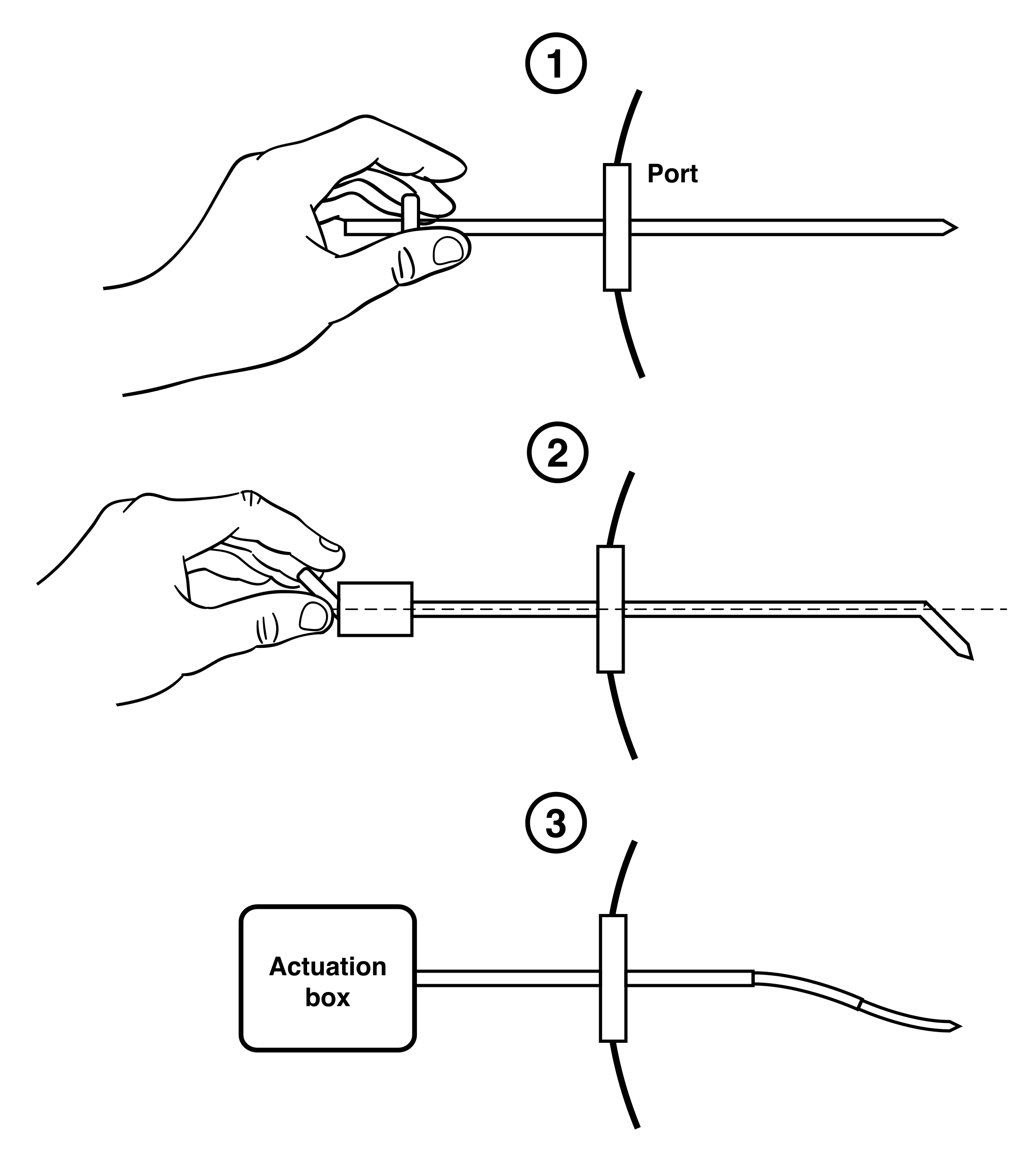
Novel chronic and acute catheter systems for direct delivery of therapeutics to the brain
Renishaw plc, Gloucestershire, UK, has produced a titanium port by metal Additive Manufacturing for use in a ground-breaking clinical trial. Manufactured on behalf of the North Bristol NHS Trust, the device enables the precise delivery of a new drug candidate, Glial Cell Line Derived Neurotrophic Factor (GDNF), directly into the brain of individuals with Parkinson’s disease, with the aim to. Renishaw plc (OTCPK:RNSHF) Q2 2021 Results Conference Call February 4, 2021 5:00 AM ET. Company Participants. Chris Pockett - Head of Communications. Will Lee - Chief Executive. The inlet port 28 and the outlet ports 25 to the pump unit 23, and the inlet/outlet port 45, 54 of the reservoir unit are, in this embodiment, in the form of a nipple over which the various tubes must be forced and subsequently compressed by tightening a ligature, a crimped ring, a deformed washer that is compressed by a threaded encircling nut.
Crossing the blood-brain barrier
Intraparenchymal drug delivery offers a practical method of bypassing the blood-brain barrier (BBB) and shows great promise in providing the next step change in the treatment of neurodegenerative, neuro oncology and other debilitating neurological conditions.
We are developing a patented range of engineering solutions to cover chronic (long term) implantable and acute (short term) implantable intraparenchymal drug delivery. Our low dead volume chronic device has a novel, MRI compatible, transcutaneous port that aims to provide a solution for simultaneous intermittent drug delivery to the central nervous system (CNS) through multiple catheters (up to four) at any time interval.
Watch an introductory video
- The neuroinfuse™ drug delivery system
A complete robotic platform
- SNO-SCIDOT Joint Conference on Therapeutic Delivery to the CNS
Features and benefits
Feature | Benefit |
| Implantation performed outside of MRI | Reduces the burden on costly MRI equipment and radiologists |
| MRI compatible | Enables real-time imaging of infusions |
| Acute infusions | Suitable for one-off infusions, catheter can be explanted after use |
| Chronic infusions | Enables repeated infusions and flexible administration regimes |
| Patient specific | Catheter length can be adjusted to encourage custom therapy distribution |
| 4-channel re-accessible catheter system with independent fluid paths and flow rates | Allows the clinician to control and customise the therapy regime |
| Repeat administrations do not require additional surgery | Reduces inherent risk of repeated surgery |
| Repeated delivery of fixed volumes | Aims to deliver consistent and repeatable treatment |
| Outpatient infusions | Reduces strain on in-patient facilities |
Working with clinical partners
Over several years Renishaw has been working with experienced clinical experts to produce a specification for an intraparenchymal drug delivery device and stereotactic delivery platform that facilitates convection enhanced delivery (CED) and other infusion applications.
The chronic product is currently undergoing clinical investigation as part of the Horizon 2020 funded TreatER project. TreatER is a first-in-human clinical study examining the intraparenchymal delivery of CDNF for the treatment of Parkinson's disease.
Drug development opportunities

At present, the Renishaw neuroinfuse chronic and acute drug delivery system can only be used in the setting of an approved clinical trial.
Renishaw is currently seeking academic, clinical and commercial partners across a wide range of indications, from oncology to neurodegenerative diseases.
The purpose of this website is only to obtain partners and not to make the device generally available.

Downloads
Renishaw Neuro Solutions Limited legal and Privacy Notices. Click here
DUBLIN--(BUSINESS WIRE)--The 'Global Orthopedic 3D Printed Devices Market 2020-2024' report has been added to ResearchAndMarkets.com's offering.
The orthopedic 3D printed devices market is poised to grow by $ 1.49 bn during 2020-2024 progressing at a CAGR of 24% during the forecast period. The reports on the orthopedic 3D printed devices market provides a holistic analysis, market size and forecast, trends, growth drivers, and challenges, as well as vendor analysis covering around 25 vendors.
The report offers an up-to-date analysis regarding the current global market scenario, the latest trends and drivers, and the overall market environment. The market is driven by the increased demand for personalized orthopedic devices and rising cost efficiency and enhanced productivity.
The orthopedic 3D printed devices market analysis includes application segment and geographic landscapes. This study identifies the growing number of orthopedic implant surgeries as one of the prime reasons driving the orthopedic 3D printed devices market growth during the next few years.
The report presents a detailed picture of the market by the way of study, synthesis, and summation of data from multiple sources by an analysis of key parameters.
The orthopedic 3D printed devices market covers the following areas:
- Orthopedic 3D printed devices market sizing
- Orthopedic 3D printed devices market forecast
- Orthopedic 3D printed devices market industry analysis.
This robust vendor analysis is designed to help clients improve their market position, and in line with this, this report provides a detailed analysis of several leading orthopedic 3d printed devices market vendors that include 3D Systems Corp., EnvisionTEC GmBH, EOS GmbH Electro Optical Systems, General Electric Co., Materialise NV, Medtronic Plc, Renishaw Plc, SLM Solutions Group AG, Stratasys Ltd., and The ExOne Company. Also, the orthopedic 3d printed devices market analysis report includes information on upcoming trends and challenges that will influence market growth. This is to help companies strategize and leverage on all forthcoming growth opportunities.
The study was conducted using an objective combination of primary and secondary information including inputs from key participants in the industry. The report contains a comprehensive market and vendor landscape in addition to an analysis of the key vendors.
The report presents a detailed picture of the market by the way of study, synthesis, and summation of data from multiple sources by an analysis of key parameters such as profit, pricing, competition, and promotions. It presents various market facets by identifying the key industry influencers. The data presented is comprehensive, reliable, and a result of extensive research - both primary and secondary.
This market research report provides a complete competitive landscape and an in-depth vendor selection methodology and analysis using qualitative and quantitative research to forecast accurate market growth.
Key Topics Covered:
1. Executive Summary
- Market Overview
2. Market Landscape

- Market ecosystem
- Value chain analysis
3. Market Sizing
- Market definition
- Market segment analysis
- Market size 2019
- Market outlook: Forecast for 2019 - 2024
4. Five Forces Analysis
- Five forces summary
- Bargaining power of buyers
- Bargaining power of suppliers
- Threat of new entrants
- Threat of substitutes
- Threat of rivalry
- Market condition
5. Market Segmentation by Application
- Market segments
- Comparison by Application
- Orthopedic implants - Market size and forecast 2019-2024
- Surgical planning - Market size and forecast 2019-2024
- Surgical instruments - Market size and forecast 2019-2024
- Market opportunity by Application
6. Customer Landscape
7. Geographic Landscape
- Geographic segmentation
- Geographic comparison
- North America - Market size and forecast 2019-2024
- Europe - Market size and forecast 2019-2024
- APAC - Market size and forecast 2019-2024
- South America - Market size and forecast 2019-2024
- MEA - Market size and forecast 2019-2024
- Key leading countries
- Market opportunity by geography
- Market drivers
- Market challenges
- Market trends
Renishaw Plc Port Devices Driver Download

Renishaw Plc Port Devices Driver Updater
8. Vendor Landscape
- Vendor landscape
- Landscape disruption
Renishaw Plc Port Devices Drivers
9. Vendor Analysis
- Vendors covered
- Market positioning of vendors
- 3D Systems Corp.
- EnvisionTEC GmBH
- EOS GmbH Electro Optical Systems
- General Electric Co.
- Materialise NV
- Medtronic Plc
- Renishaw Plc
- SLM Solutions Group AG
- Stratasys Ltd.
- The ExOne Company
10. Appendix
Renishaw Plc Port Devices Driver Windows 7
- Scope of the report
- Currency conversion rates for US$
- Research methodology
- List of abbreviations
Renishaw Plc Port Devices Driver
For more information about this report visit https://www.researchandmarkets.com/r/ma30rb
